Creating Equations to Solve Problems. A proton is present in the nuclei of all other atoms in.
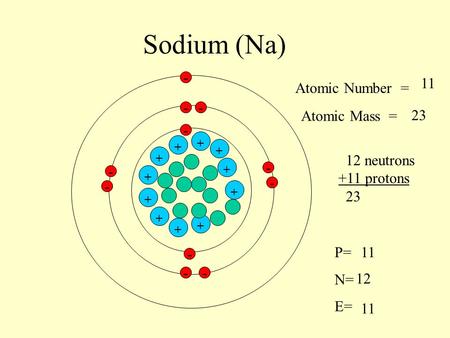
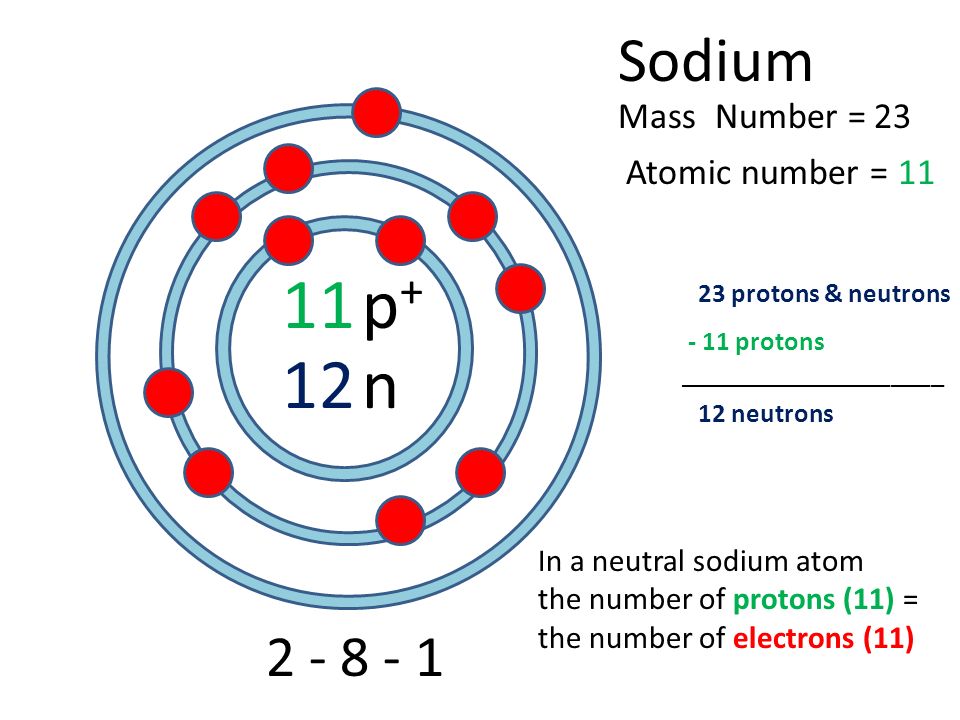
11 12 Sodium Atomic Number Mass Number P P N Protons Neutrons 12 Neutrons In A Neutral Sodium Atom The Number Of Protons 11 Ppt Download
Give the number of electrons in the species.
. Toggle Topic D Topic D. It is sealed at both the. For the following statements write T for True and F for False.
In everyday life on Earth isolated hydrogen atoms called atomic hydrogen are. Thomson proposed that the nucleus of an atom contains only nucleons. Of electrons 29 Electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1.
A hydrogen atom is an atom of the chemical element hydrogenThe electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Mass number of X protons neutrons 6 6 12 Mass number of Y 6 8 14 The species X and Y are the isotopes because their atomic numbers are same and their mass numbers are different. Toggle Topic A Topic A.
He performed the experiment in a discharge tube which is a cylindrical hard glass tube about 60 cm in length. Shapes and Centers of. An atom of an element contains 29 electrons and 35 neutrons.
Structure of Atom Class 11 Notes Chemistry Chapter 2 Discovery of ElectronDischarge Tube Experiment In 1879 William Crooks studied the conduction of electricity through gases at low pressure. Toggle Module 2 Module 2. Deduce i the number of protons and ii the electronic configuration of the element.
The discovery of the proton is credited to Ernest Rutherford who proved that the nucleus of the hydrogen atom ie. How were Neutrons Discovered. Atomic hydrogen constitutes about 75 of the baryonic mass of the universe.
Of protons in a neutral atom No. H 2 H 2.
If An Atom Contains 12 Protons 12 Electrons And 12 Neutrons Then What Is Its Atomic Number And Mass Number Quora

11 12 Sodium Atomic Number Mass Number P P N Protons Neutrons 12 Neutrons In A Neutral Sodium Atom The Number Of Protons 11 Ppt Download
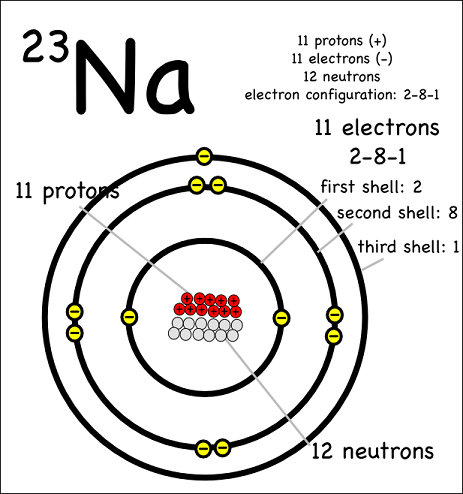
0 Comments